Pfizer said it has selected lower dosages of its COVID-19 vaccine for Phase 2/3 portions of its clinical trial involving kids younger than 11 than the volume given to shot recipients ages 12 and older.
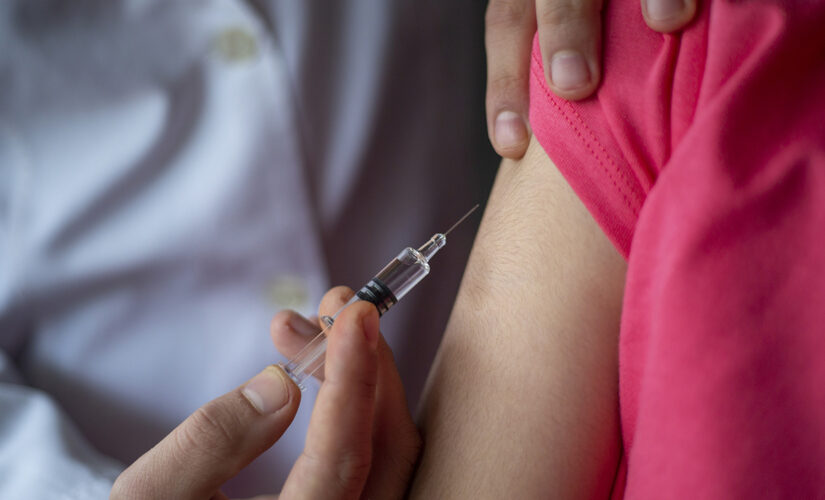

Pfizer said it has selected lower dosages of its COVID-19 vaccine for Phase 2/3 portions of its clinical trial involving kids younger than 11 than the volume given to shot recipients ages 12 and older.