NEWYou can now listen to Fox News articles!
The U.S. Food and Drug Administration issued a rule Tuesday to allow certain types of hearing aids to be made available over-the-counter without the need for a prescription or medical examination.
The FDA said the rule, which will create a new regulatory category for over-the-counter hearing aids, applies to those “with perceived mild to moderate hearing impairment.“
“In creating a regulatory category for OTC hearing aids and amending existing rules, we intend to provide reasonable assurance of safety and effectiveness for these devices as well as foster access to, and innovation in, hearing aid technology, thereby protecting and promoting the public health,” the rule says.
The FDA claims that the rule will lower the costs of hearing aids as well. It follows President Biden’s July 2021 Executive Order on Promoting Competition in the American Economy, which specifically said that the Secretary of Health and Human Services should publish “a proposed rule on over-the-counter hearing aids.”
“It’s the latest action we are taking to make our economy more competitive and less concentrated,” Biden said in a statement on Tuesday. “When too few companies dominate, American consumers pay higher costs. We’re finally building an economy that works for working families.”
FDA ISSUES PROPOSAL TO CREATE NEW CATEGORY OF OVER-THE-COUNTER-HEARING AIDS
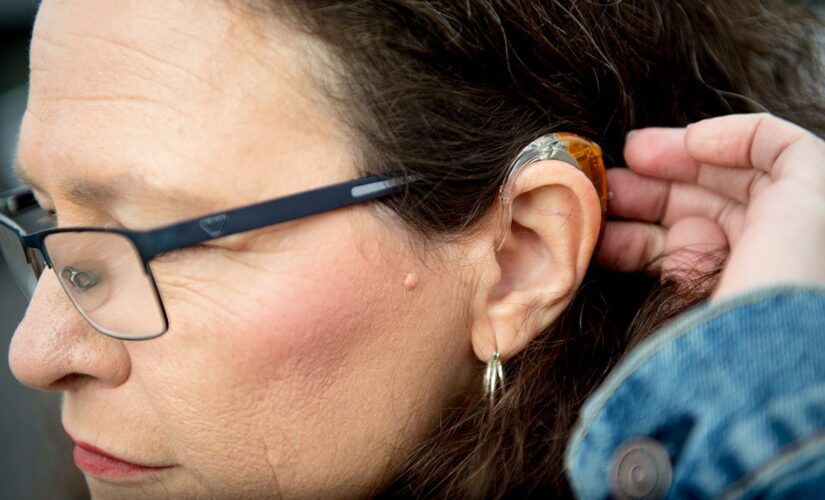
Kim M. Smith, leader of the Utah Deaf Hospital Rights movement and president of the Utah Association of the Deaf, brushes her hair away from her hearing aid as she poses for a portrait Monday, Jan. 20, 2020, at Alta View Hospital in Sandy, Utah.
(Isaac Hale/The Daily Herald via AP)
The final version of the rule came after a notice-and-comment period that yielded more than 1,000 public comments from hearing aid manufacturers and consumers, as well as professional associations, lawmakers, and state agencies, the FDA said. This resulted in changes to the proposed rule, such as lowering the maximum sound output for OTC hearing aids and requiring an adjustable volume control.
The FDA said that the rule will go into effect in mid-October and that Americans will be able to find over-the-counter hearing aids in drug stores and other retail stores – including online – at that time.
The Food and Drug Administration issued a proposal Tuesday in an effort to provide over-the-counter hearing aids.
(iStock)
“Reducing health care costs in America has been a priority of mine since Day One and this rule is expected to help us achieve quality, affordable health care access for millions of Americans in need,” Health and Human Services Secretary Xavier Becerra said in a statement. “Today’s action by the FDA represents a significant milestone in making hearing aids more cost-effective and accessible.”
CLICK HERE TO GET THE FOX NEWS APP
The FDA specified that the new category of OTC devices “applies to certain air-conduction hearing aids intended for people 18 years of age and older who have perceived mild to moderate hearing impairment.” Anything else, be it a different type of aid or one for someone younger, would still require a prescription.