The Food and Drug Administration (FDA) took action Friday to expand the use of the antiviral remdesivir drug Veklury to certain non-hospitalized adults and pediatric patients for the treatment of mild-to-moderate COVID-19 disease.
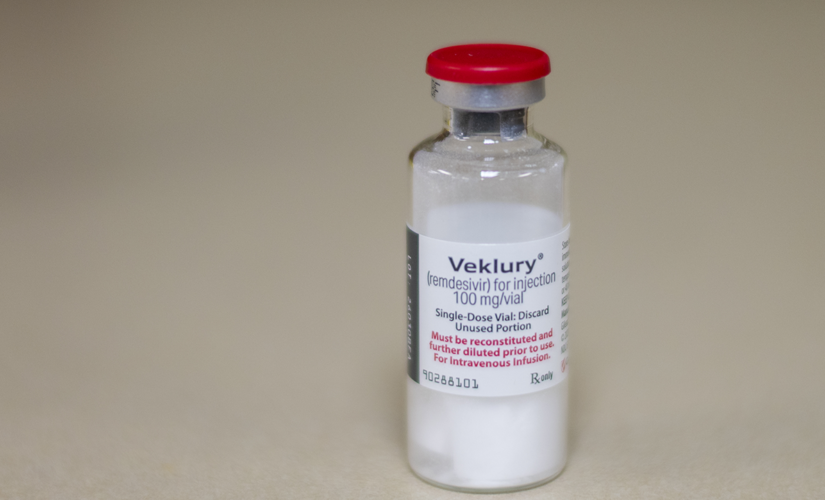

The Food and Drug Administration (FDA) took action Friday to expand the use of the antiviral remdesivir drug Veklury to certain non-hospitalized adults and pediatric patients for the treatment of mild-to-moderate COVID-19 disease.