The U.S. Food and Drug Administration (FDA) announced Thursday that Omicron-targeting bivalent COVID-19 vaccines have been authorized for use in children as young as 6 months old.
“Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age,” the agency said in a statement.
The agency said children ages 6 months through 5 years who have received the original Moderna COVID-19 vaccine are now eligible to receive the updated booster shot which specifically targets the Omicron variant of the virus.
Children who have not yet begun the three-dose series of the Pfizer-BioNTech vaccine or have not yet received a third dose, will now be given the bivalent booster as their third shot following two doses of the original Pfizer COVID-19 vaccine.
US PLANS END TO MONKEYPOX PUBLIC HEALTH EMERGENCY IN JANUARY
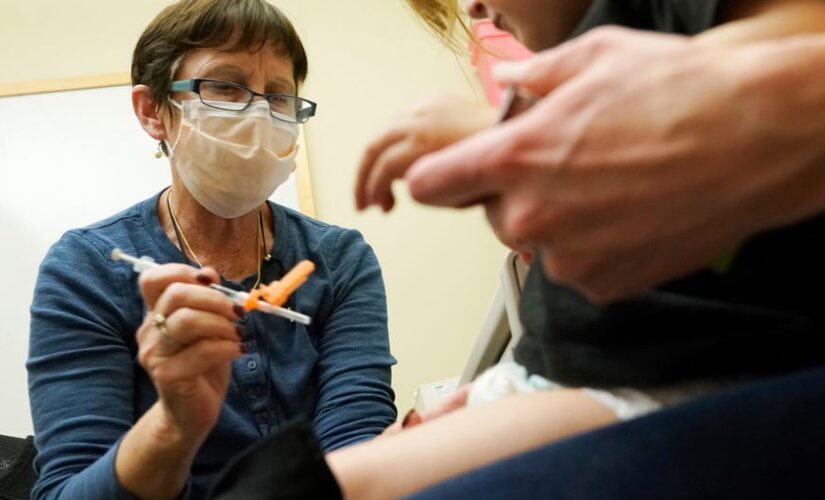
Deborah Sampson, left, a nurse at a University of Washington Medical Center clinic in Seattle, gives a Pfizer COVID-19 vaccine shot to a 20-month-old child, June 21, 2022, in Seattle.
(AP Photo/Ted S. Warren, file)
Kids ages 6 months through 4 years who have already completed their three-dose Pfizer series are not eligible for the updated booster shot, the FDA said.
The decision comes three days after Pfizer and BioNTech submitted an application to the FDA for an emergency use authorization for their Omicron BA.4/BA.5-Adapted Bivalent COVID-19 vaccine.
“More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to consider doing so – especially as we head into the holidays and winter months where more time will be spent indoors,” said FDA Commissioner Robert M. Califf, M.D., in a statement.
“As this virus has changed, and immunity from previous COVID-19 vaccination wanes, the more people who keep up to date on COVID-19 vaccinations, the more benefit there will be for individuals, families and public health by helping prevent severe illnesses, hospitalizations, and deaths,” Califf added.
US HOSPITALIZATIONS RISE AS RESPIRATORY VIRUSES CONTINUE TO SPREAD: REPORT
Sign is seen outside the Food and Drug Administration headquarters in White Oak, Maryland, Aug. 29, 2020.
(Reuters/Andrew Kelly/File Photo)
The U.S. Centers for Disease Control and Prevention is expected to follow suit and recommend the bivalent boosters for young children.
Just 3% of children under 2 and nearly 5% of those between the ages of 2 to 4 have gotten their primary doses so far, according to the CDC.
“Vaccines remain the best defense against the most devastating consequences of disease caused by the currently circulating omicron variant,” FDA vaccine chief Dr. Peter Marks said in a statement.
The updated vaccines from Moderna and Pfizer are combination shots, containing half the original vaccine and half tweaked to match the BA.4 and BA.5 Omicron strains that until recently were dominant. Now, BA.5 descendants are responsible for most COVID-19 cases.
ARMY IS ONLY SERVICE BRANCH BOOTING TROOPS OVER COVID VACCINE MANDATE IN FULL FORCE
This August 2022 photo provided by Pfizer shows vials of the company’s updated COVID-19 vaccine during production in Kalamazoo, Michigan
(Pfizer via AP)
The CDC last month released the first real-world data showing that an updated booster, using either company’s version, does offer added protection to adults. The analysis found the greatest benefit was in people who had never had a prior booster, just two doses of the original COVID-19 vaccine — but that even those who had a summer-time dose were more protected than if they had skipped the newest shot.
Pfizer and Moderna executives thanked the FDA for expanding the authorization for their vaccines.
CLICK HERE TO GET THE FOX NEWS APP
“With the FDA’s decision, children and adolescents of all age groups in the U.S. will now be eligible for our updated bivalent COVID-19 booster, providing families with an important protective tool as we continue through the winter months,” said St?phane Bancel, CEO of Moderna. “We appreciate the FDA’s timely review.”
“This authorization offers an opportunity for parents to help better protect their young children against COVID-19, including disease caused by Omicron sublineages,” said Pfizer CEO Albert Bourla. “Nearly 40 million Americans have received a booster dose of an updated vaccine. It is critical that we all continue to do our part to help protect ourselves by staying up to date with COVID-19 vaccinations, as recommended by public health authorities, especially now as we plan to gather for the holidays and head into the winter season.”
The Associated Press contributed to this report.