Last week’s White House report reiterated President Biden’s employer mandate that businesses with 100 or more employees require every worker to be fully vaccinated for COVID-19 or tested weekly. The requirement impacts more than 80 million workers in the private sector.
Jeffrey Zients, the White House COVID-19 response coordinator, summarized in last week’s press briefing that, “We are on track to quadruple the supply of rapid, at-home tests available to Americans by December to more than 200 million a month and to increase the number of places Americans can access free testing in the United States to 30,000 community-based locations.”
He emphasized the president’s staunch commitment in adding $1 billion of extra funding already to the recent $2 billion investment to increase supply.
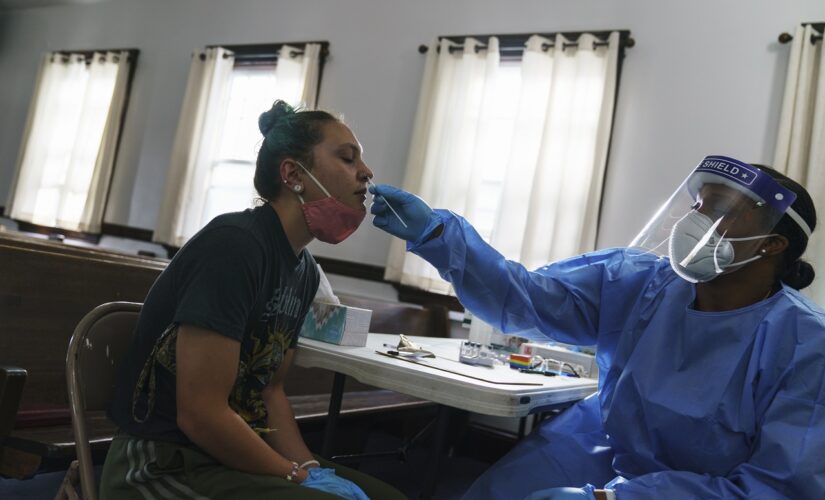
Jessica Leo, left, is tested for COVID-19 at a clinic set up at Bethel AME Church on Sept. 24, 2021, in Providence, Rhode Island.
(AP)
CORONAVIRUS IN THE US: STATE-BY-STATE BREAKDOWN
Two manufactures, Quidel and OraSure, will further expand their production capabilities of rapid tests, which is expected to increase tens of millions of tests per month.
“Employer demand has gone crazy,” the Quidel Chief Executive Doug Bryant said to Reuters recently, noting as of last week the company was unable to meet all the employer requests.
The Food and Drug Administration (FDA) approved a new rapid at-home test produced by ACON laboratories known as Flowflex, further accelerating the availability of at-home testing. Zients considered the test the most affordable on the market, estimating the kit will retail for about $10 per test.
He noted the large government purchases of these at-home tests should drive down costs. In addition, the White House worked with Amazon, Kroger and Walmart to sell rapid test kits at cost for the next several months.
Bidding wars between health systems and states have so far lead to higher price points in the U.S, according to Reuters.
A nurse holds a coronavirus test kit in a laboratory. (iStock)
(iStock)
Dr. Arthur Reingold, chair of California’s COVID-19 Scientific Safety Review Workgroup and division head of epidemiology at UC Berkeley, cautioned in an email to Fox News: “The U.S. has lagged behind many other wealthy countries in making reliable COVID-19 tests, especially rapid tests, widely available.”
WHY ARE SOME COUNTRIES RECOMMENDING A SINGLE CORONAVIRUS VACCINE DOSE FOR FOR TEENS, YOUNG ADULTS?
While European countries continued to purchase rapid test kits back in May, the laboratory Abbott, which makes one of the most popular rapid testing kits known as BinaxNOW, decided to lay off employees and throw out expired components because of its surplus around that time, according to Newsy.
On Oct. 5, the FDA issued an alert detailing a recall of the COVID-19 rapid testing kits produced by the Australian-based company Ellume after discovering some of the tests resulted in a higher false-positive rate of COVID-19 than expected. A false positive result means the patient is diagnosed with an infection that they don’t actually have.
However, Dr. Jeffrey E. Shuren, director of FDA’s Center for Devices and Radiological Health, wrote in a press statement earlier this month that at-home COVID-19 tests are a high priority for the FDA to review.
Reingold noted, “The announcement that the Biden administration is directing more funding toward making rapid tests more widely available is very welcome news. The results of such tests can facilitate the decisions of individuals and families; schools and workplaces; and everything from long-term care facilities to restaurants to sports clubs to spectator events and concerts concerning the safe return to the activities that everyone is eager to resume.”
The White House last week doubled the number of local pharmacies in the federal government’s free testing program, so there are around 30,000 sites available to access a free COVID-19 rapid test.
Large pharmacy chains are mobilizing into action to meet the surging demand. Joseph Goode, senior director of corporate communications at CVS, told Fox News: “We continue to be able to meet the demand for COVID-19 testing in most locations, even with increasing numbers of patients seeking out tests. In addition, CVS stores across the country offer multiple over-the-counter COVID-19 test kits, allowing patients to self-test at home.”
In response to the high demand, CVS introduced product limits of Abbott’s BinaxNOW and Quidel tests, but Goode noted CVS is committed to work with suppliers to meet customer demand.